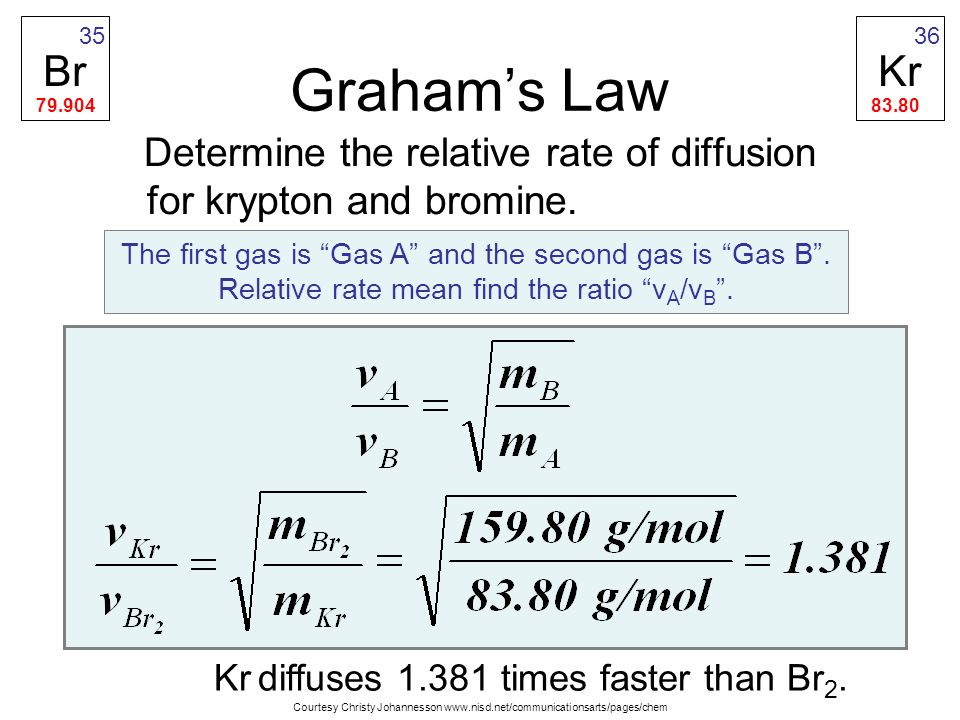
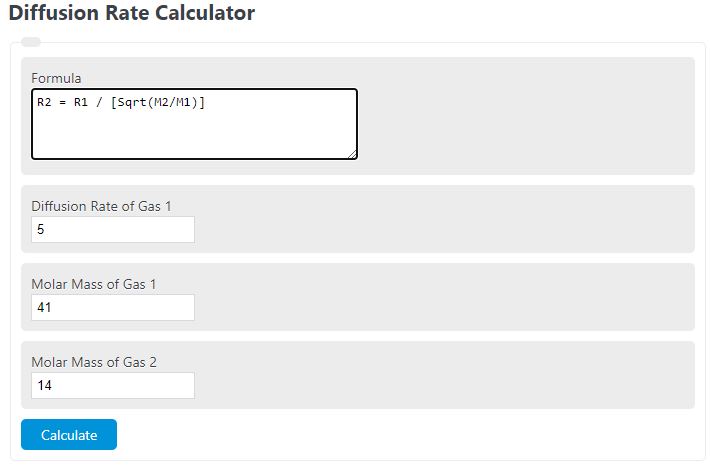
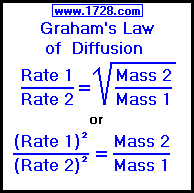
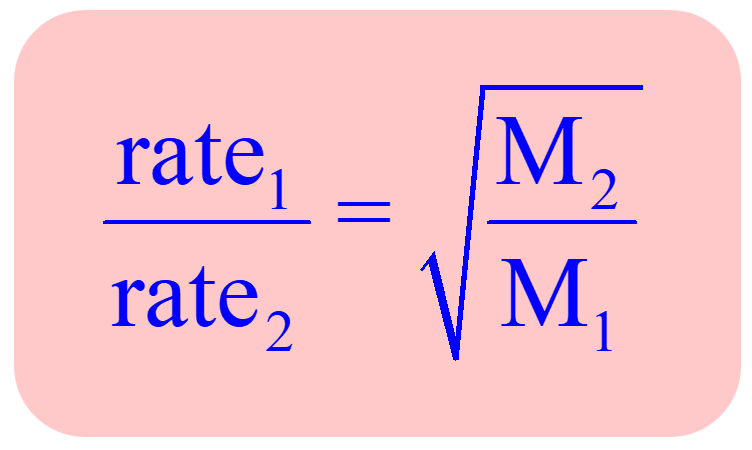50 ml of a gas A diffuse through a membrane in the same time as for the diffusion of 40 ml of a gas B under identical pressure - temperature conditions. If
Rate of diffusion of a saturated hydrocarbon is about 1/6 th of that of hydrogen under similar conditions of temperature and pressure. What is the molecular formula of that hydrocarbon?

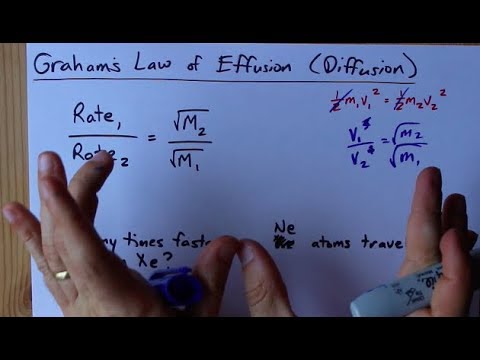
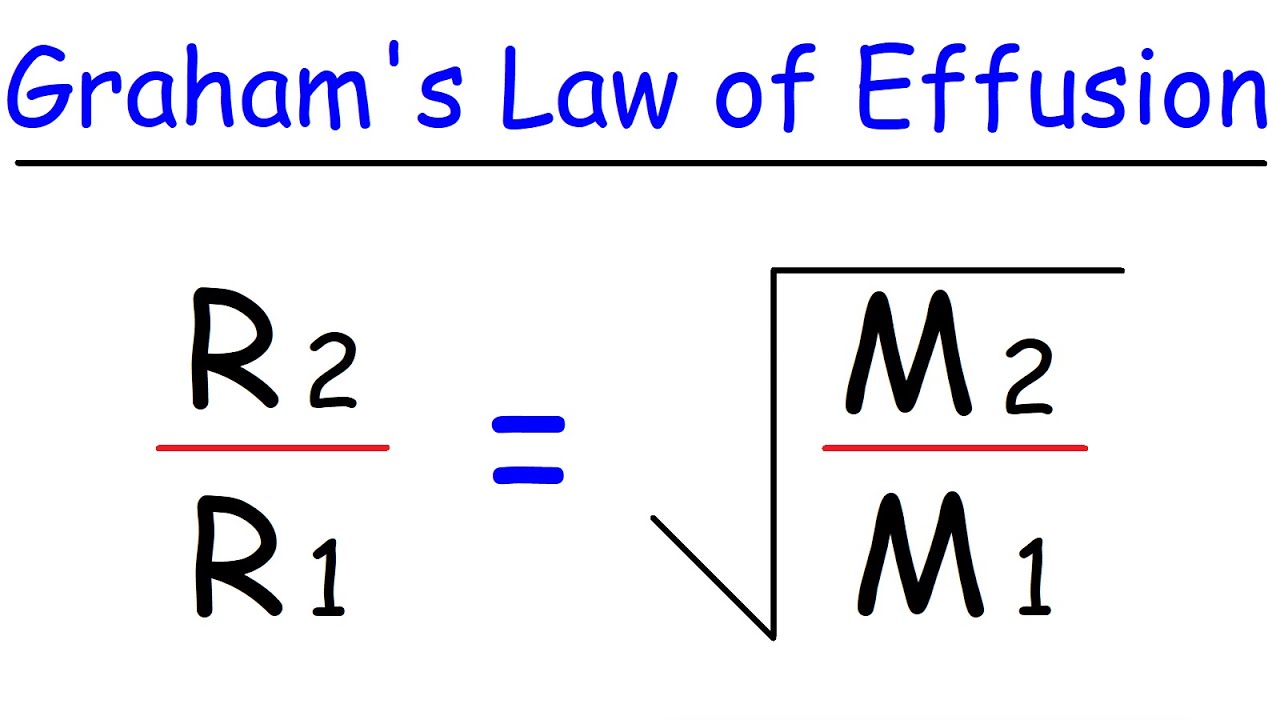
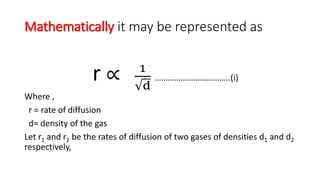
Graham's Law of Diffusion Graham's Law KE = ½mv 2 Speed of diffusion/ effusion –Kinetic energy is determined by the temperature of the gas. –At the same. - ppt download