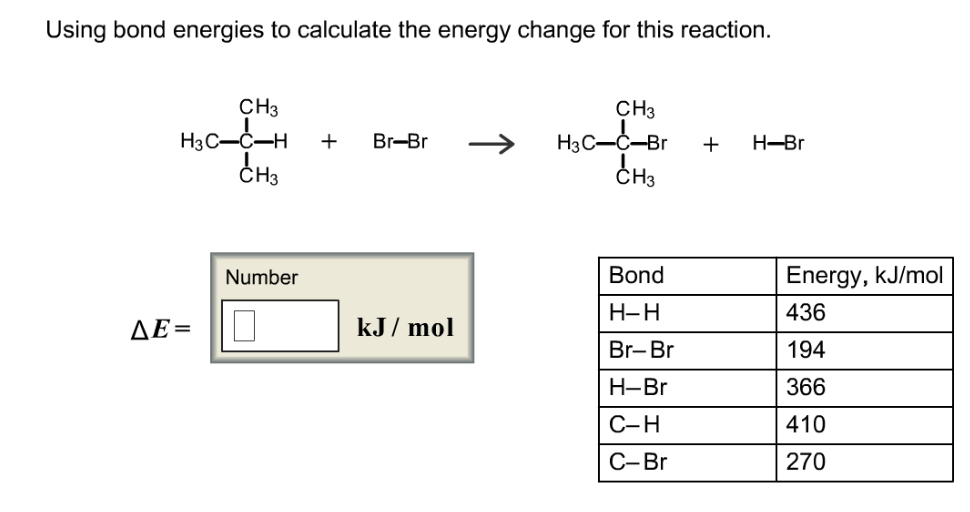
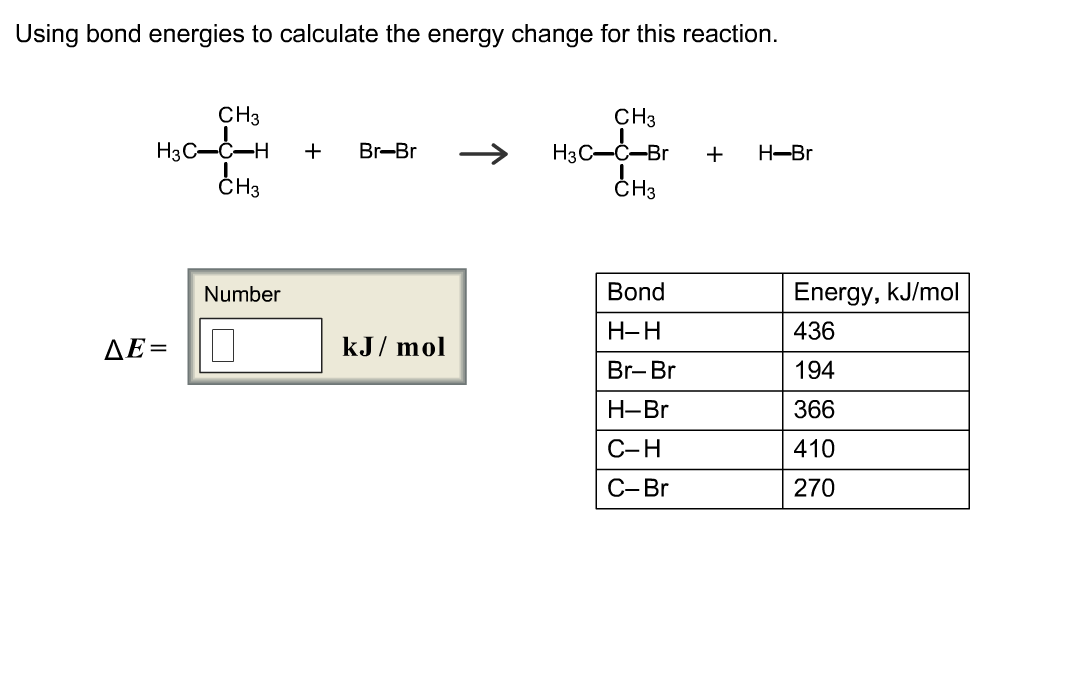
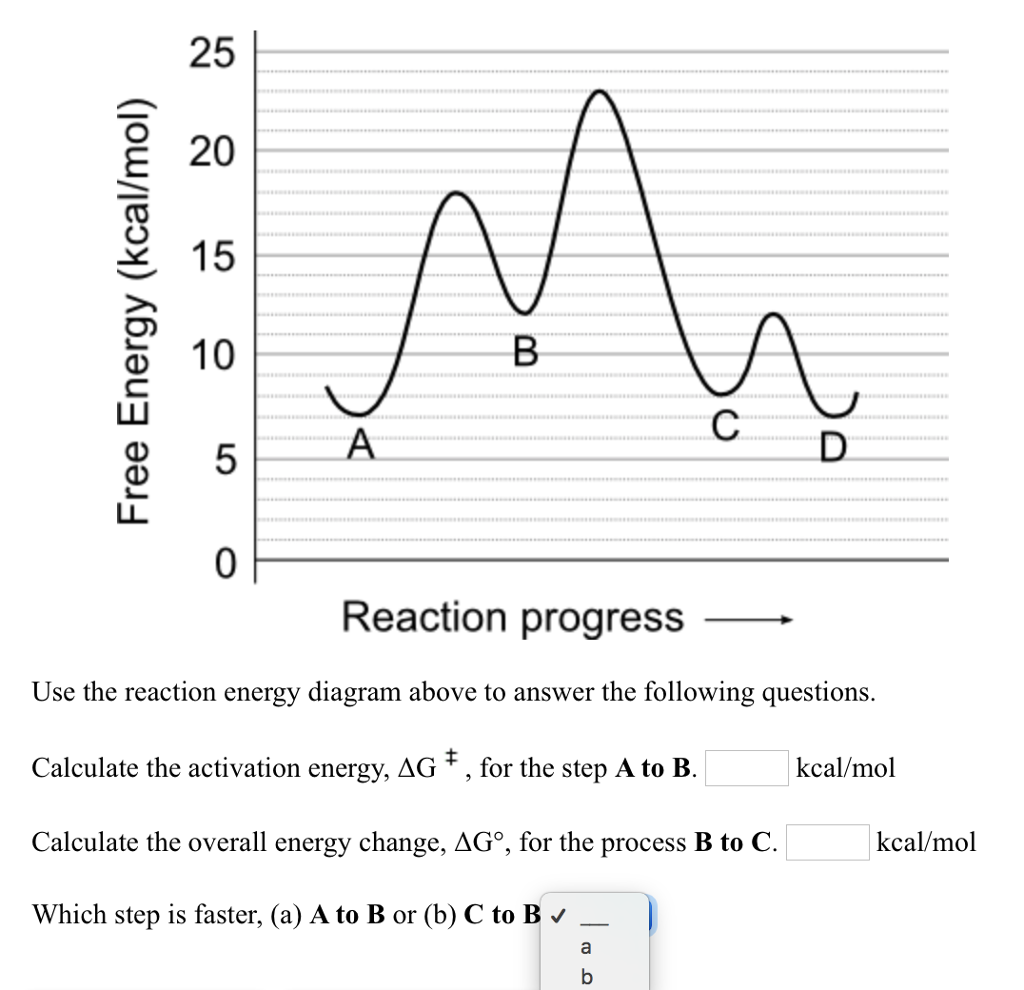
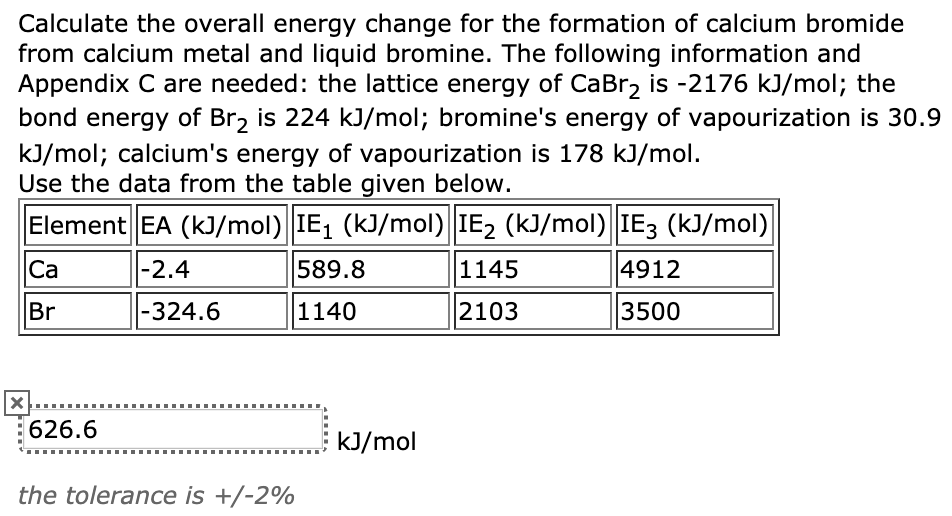
Determine the overall energy change for the reaction between hydrogen and oxygen shown in Question - Brainly.com
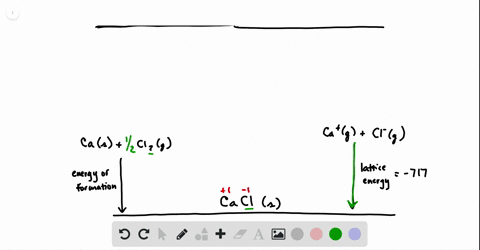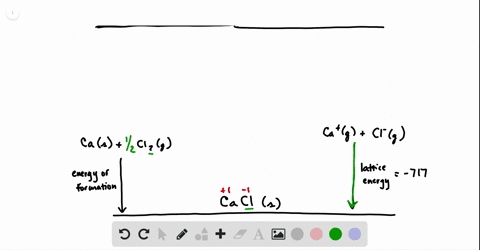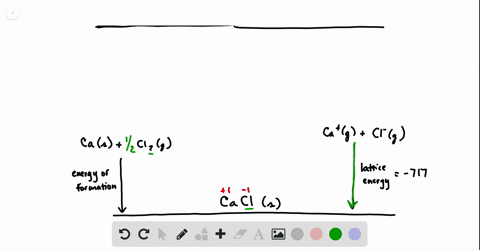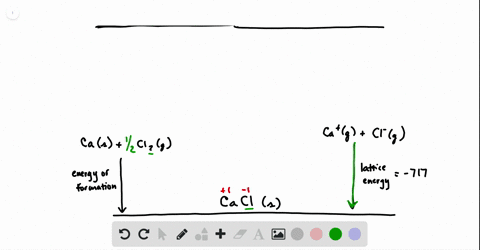
SOLVED:Calculate the overall energy change in kilo joules per mole for the formation of CaCl(s) from the elements. The following data are needed: E ea for Cl(g)=-348.6 kJ / mol Ei 1
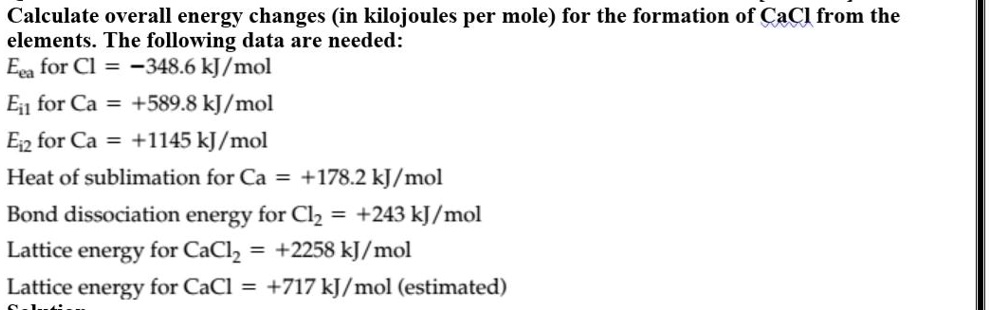
SOLVED: Calculate overall energy changes (in kilojoules per mole) for the formation of CaCl from the elements. The following data are needed: Eua for CI -348.6 kJ / mol Eil for Ca +